Student-Led Research Reveals “Off-Switch” for Autophagy
New discovery came from chance occurrence in undergraduate teaching lab
A chance observation in an undergraduate laboratory class has shed light on a key cleaning and recycling process carried out by all eukaryotic cells. Autophagy breaks down organelles, proteins and other molecules so their components can be reused and plays a protective role in preventing disease. However, when autophagy doesn’t work correctly, it’s associated with cancer and neurodegenerative diseases including Alzheimer’s. Previous research has uncovered how cells activate autophagy, but little is known about how it is switched off.
The new research, published Dec. 14 in Molecular Biology of the Cell, shows that cells switch off autophagy by rearranging structural proteins called septins. The researchers showed that yeast that lacked functional septins could activate autophagy but were unable to stop it.
“Our study has opened a new way to think about how autophagy can be turned off, which is something biologists have very little insight on,” said senior author Kenneth Kaplan, a professor of molecular and cellular biology. “This has important implications for understanding autophagy’s protective role in various disease progressions, and how it becomes dysregulated in other diseases.”
The study is also an example of the value of including undergraduates in research, for both students and the advancement of our collective understanding of basic science. All phases of the study were undergraduate-driven, and all three of Kaplan’s co-authors, Luis Perucho-Jaimes, Jonathan Do, and Alexandria Van Elgort, worked on the study as undergraduate research assistants.
“This paper really illustrates the serendipity of combining experiential undergraduate education with basic research,” said Kaplan. “It was an undergrad-driven project and I was really proud of them for pushing all of this forward.”

A chance observation
The study arose from a student’s observation in Kaplan’s 2016 cell biology lab class. Due to delays caused by what Kaplan describes as “class chaos,” yeast cells that the students were imaging entered a nutrient deprived state by the time they made it to the microscope.
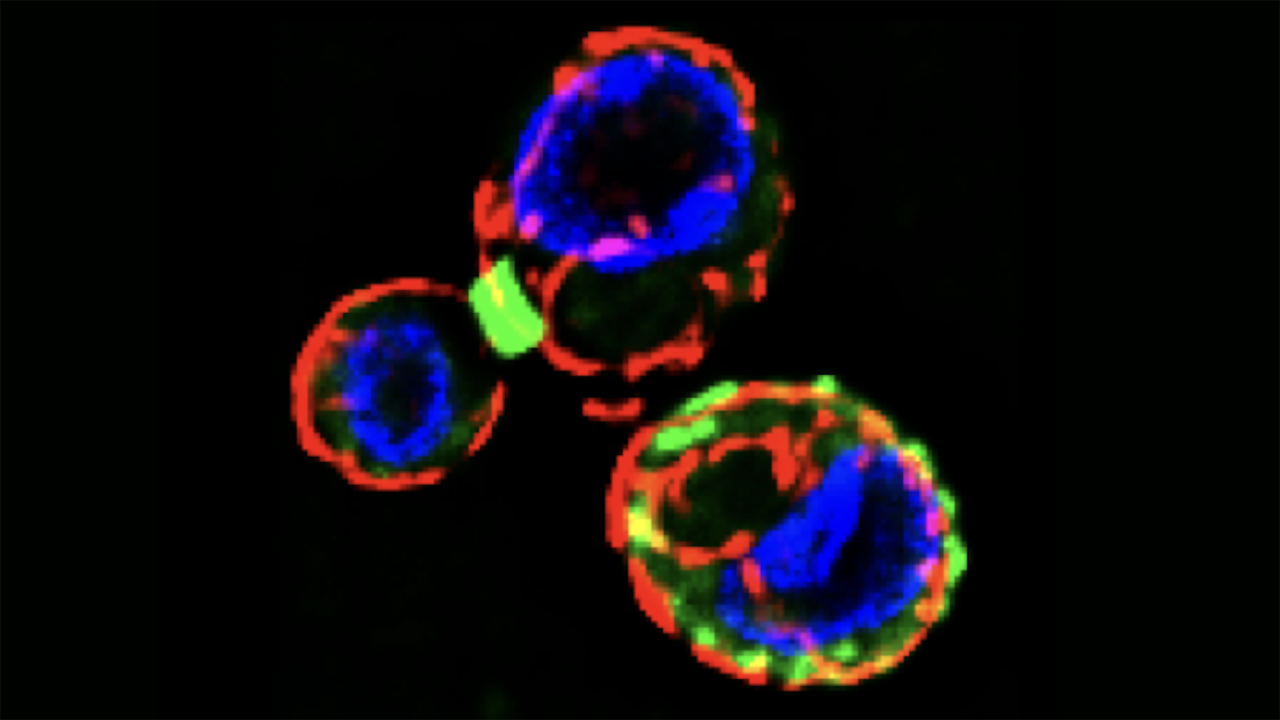
Under the microscope, one student, Jonathan Mendez, noticed that the cells’ cytoskeletons had undergone a surprising rearrangement. Specifically, the septins—filamentous proteins usually involved in cell division—had rearranged themselves into an unusual and previously undocumented configuration.
When yeast cells divide, septins usually form a ring at the “neck” that divides the mother and daughter cells. However, in the starved yeast cells, septins formed localized patches that were scattered around the cell membrane. Initially, Kaplan and his students were unsure what this observation could mean. Mendez pushed the group to investigate further, so Kaplan took the question back to the lab.
“Jonathan definitely spurred this on—I think without his inquisitiveness we probably would have just said ‘who knows what happened’ and moved on,” said Kaplan.

Digging deeper
Knowing that the cells must have entered starvation mode, Kaplan speculated that this rearrangement might have something to do with autophagy.
“Autophagy is one of the key responses to starvation—the cell essentially degrades some of its own structures in order to survive” Kaplan said.
However, it wasn’t clear whether the septins were involved in switching autophagy on or off. To test these two possibilities, the team used high-resolution fluorescence microscopy to make time-lapse videos of yeast cells before and after they were subjected to starvation conditions to examine the timing of the septin rearrangement. These experiments revealed that septin reorganization occurred after autophagy was activated, indicating that the septin patches are not involved in switching autophagy on.
When the researchers measured autophagy rates and duration in “septin mutant” strains of yeast that are unable to produce septin, they found that the septin-less yeast performed higher rates of autophagy than wild-type yeast, and also continued to perform autophagy long after wild type cells had switched the process off.
“Instead of turning autophagy off after 24 hours like most wild type cells, in septin mutants we saw that autophagy persists for quite a bit longer,” said Kaplan. “It seems pretty clear that without septins, autophagy fails to shut itself off.”
This finding offers one of the first clues as to how cells downregulate autophagy. For Kaplan, it’s opened new potential avenues of study, including research into neurodegenerative diseases.
“This work has led my lab to start a longer-term effort to apply our findings to the cell biology of neurodegenerative disease and the connection between septins and dysfunctional neurons,” he said.
Luis Perucho-Jaimes is now working at UC Davis; Jonathan Do is pursuing studies at the University of Arizona; and Alexandria Van Elgort is pursuing studies at Stanford Medicine.
Media Resources
- Septins modulate the autophagy response after nutrient starvation | Molecular Biology of the Cell (molbiolcell.org)
- Liana Wait is a freelance science writer based in Philadelphia. She has a Ph.D. in ecology and evolutionary biology and specializes in writing about the life sciences.