Developing and Testing Genome Editing Technology for Human Health
We might one day be able to treat diseases or inherited disorders by rewriting parts of the genetic code in our own cells. The National Institutes of Health established the Somatic Cell Genome Editing consortium, with funding of $190 million over six years, to advance research in genome editing, develop tools and test them in animal models before advancing to human clinical trials.
A perspective article published in Nature on April 7 reviews the field and sets out the goals and priorities of the consortium. Among the authors are Professor R. Holland Cheng, Department of Molecular and Cellular Biology, UC Davis College of Biological Sciences; Professors Kit Lam and David Segal, Department of Biochemistry and Molecular Medicine, UC Davis School of Medicine; and Professor Alice Tarantal, California National Primate Research Center and Department of Pediatrics, UC Davis School of Medicine.
Somatic cells are all the cells of the body – liver, skin, muscle, brain – other than the germline cells that give rise to sperm and eggs. Edits made to somatic cells cannot be carried over to offspring.
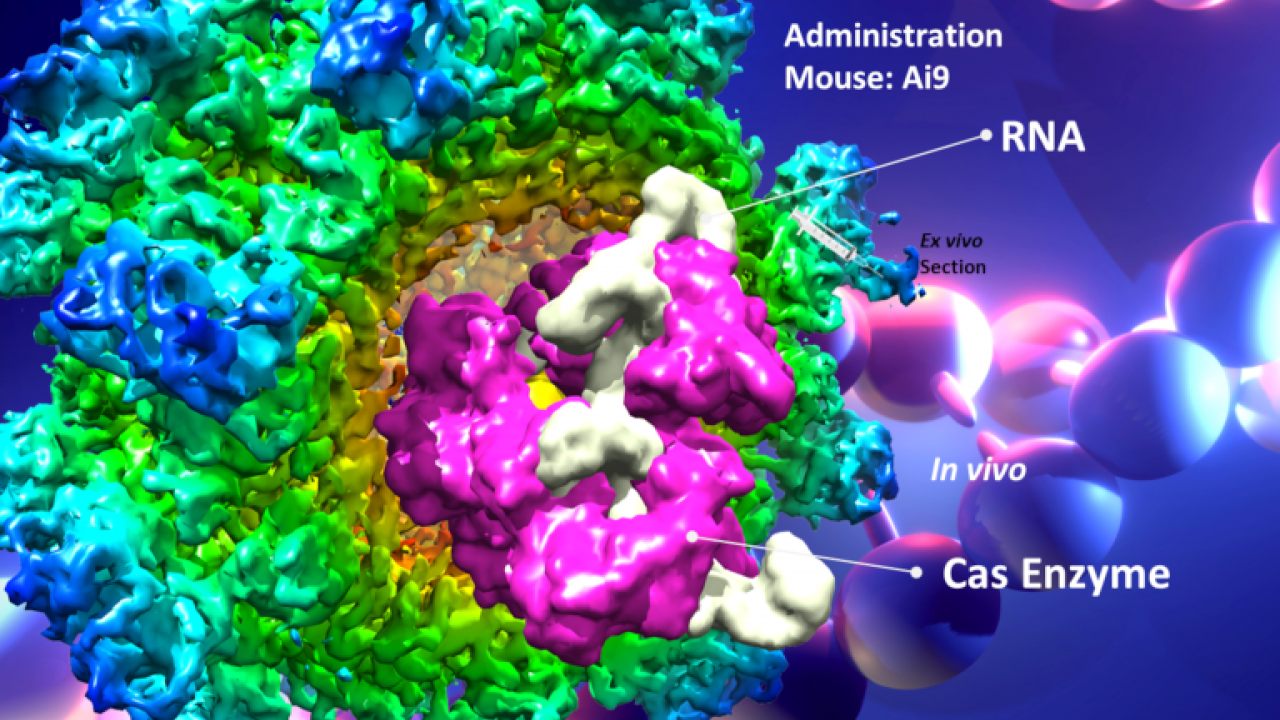
The tools that might be used to edit DNA inside human cells include CRISPR-Cas9 as well as other editing technologies. But just as important are the technologies that package gene-editing tools, so they can travel into the body and into cells.
UC Davis develops oral delivery technology
Among those tools is a system developed by Cheng, Lam, Segal, and colleagues based on an engineered, non-infectious hepatitis E capsid. The goal is to be able to deliver on-target therapies in forms of encapsulated proteins or nucleotides by mouth that would enter the body through the gut. It is the main oral delivery system being developed successfully on mucosal routes and is due to enter national animal trials by the NIH consortium, Cheng said.
A final step before genome editing therapies can enter human clinical trials is to test them in animal models. These experiments, initially in mice and later in large animals such as pigs and non-human primates, are essential to show that genome editing therapies find their way to the right cells and tissues, that they are effective, and that they do not cause unexpected or off-target effects. In 2019, the NIH awarded a grant of $9 million to UC Davis to establish the Nonhuman Primate Testing Center for Evaluation of Somatic Cell Genome Editing Tools at the California National Primate Research Center.
Media Resources
- Read the paper in Nature
- UC Davis launches center to advance genome-editing tools (UC Davis Health)
- UC Davis awarded new CRISPR grant to test novel approach to cancer treatment (UC Davis Health)
