
Cell Biologist Elected as a Fellow of The Royal Society
Neil Hunter recognized for discoveries key to fertility, heredity and evolution
Neil Hunter, a professor in the Department of Microbiology and Molecular Genetics and an Investigator with the Howard Hughes Medical Institute, has been elected as a Fellow of The Royal Society in London.
This prestigious appointment, announced May 20, was offered to only 70 scientists across the world.
“I’m thrilled and surprised,” said Hunter. “The acknowledgment means a great deal to me, because it’s an election by some of the best scientists in the world.”
“I’m especially thankful for the amazing people who have worked in my lab over the years,” he added. “Everything that we’ve accomplished has depended on having students and collaborators who were highly talented and motivated.”
Hunter was elected for his studies of homologous recombination — a chromosome repair process that lies at the heart of sexual reproduction, evolution and natural selection. Understanding how this process works at the molecular level — and how it is affected by a person’s health, age and environment — could lead to improved treatment for fertility disorders, and enhanced techniques for breeding and genetic engineering plants and animals.
Reshuffling the genetic deck
Homologous recombination is indispensable for heredity. When you meet a family with multiple children, it can be striking to see how each child is a unique blend of the parents. This mixing of traits happens because the genetic deck is shuffled during reproduction.
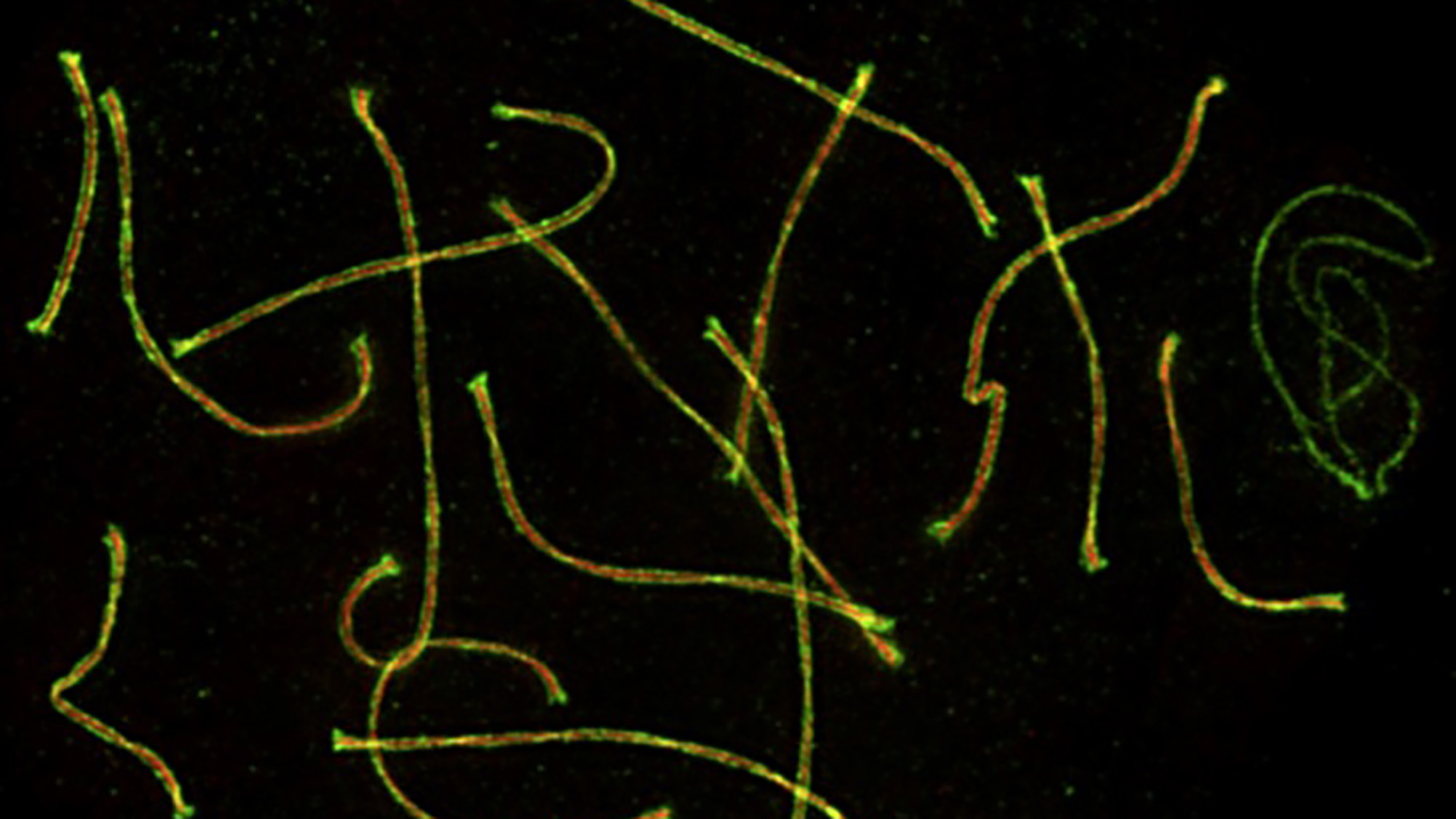
It ensures that each new generation will include individuals with different gene combinations compared to their parents. This genetic diversity helps maximize the ability to survive and adapt in a changing environment.
Recombining of chromosomes happens during meiosis, a specialized cell division that occurs in the testes and ovaries to produce sperm and eggs.
We have 46 chromosomes in each of our cells – 23 pairs of matching, “homologous” chromosomes – with one of each pair inherited from each of our parents. To make equal contributions of genetic material to their offspring, each parent will pass only 23 chromosomes to their child, through the egg and sperm. Each of those 23 is a shuffled mix of the pair that came from the child’s grandparents.
The shuffling of DNA happens during a special stage of meiosis. The chromosomes line up precisely into 23 pairs, and undergo homologous recombination – that is, they swap segments of DNA along their length.
Apportioning chromosomes
This process of homologous recombination doesn’t just shuffle the genes. It also performs another crucial function. It creates connections between matching chromosomes – called crossovers – that are critical for correctly apportioning a single set of 23 chromosomes to each sperm or egg. Maintaining these connections is especially critical in women, in whom the process of recombination in egg cells begins during fetal development, but then pauses around the time of birth. Those connections between chromosomes then have to survive for decades, until a given egg is ovulated and chromosomes are finally apportioned.
While recombination is essential for reproduction, “it’s a double-edged sword and can be dangerous,” said Hunter. To start the process of homologous recombination, cells undergoing meiosis make hundreds of breaks in their DNA molecules — “self-inflected DNA damage,” he said — similar to the DNA breaks that occur with radiation exposure.
The cell then repairs those DNA breaks via homologous recombination, repairing the broken ends by splicing a segment of DNA from one chromosome onto its matching partner.
“Bad things can happen if the recombination goes awry,” said Hunter. It can cause sperm and eggs to die, or produce cells with chromosomal abnormalities or mutated genes that can cause infertility, miscarriage or congenital diseases.
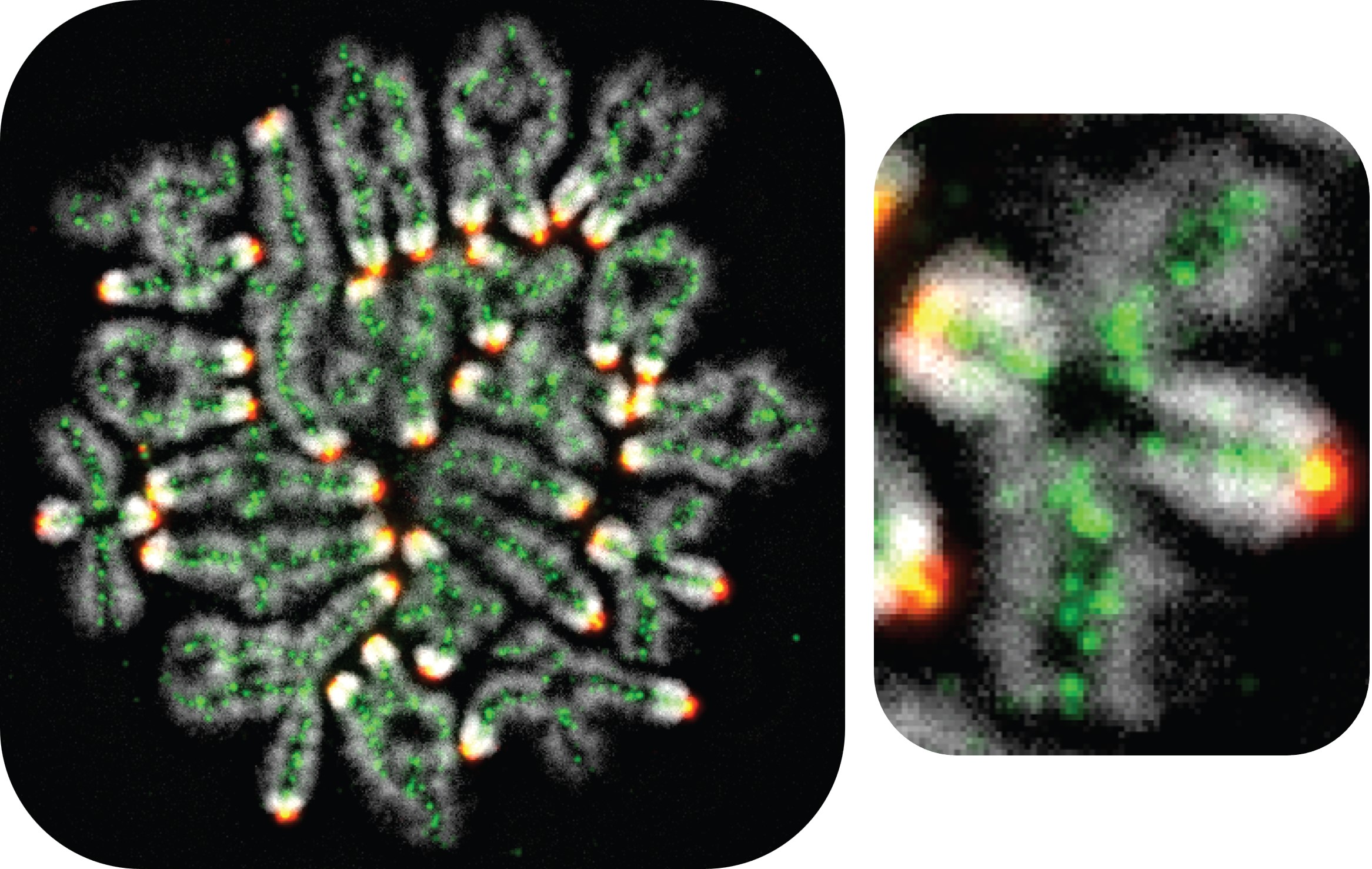
The inner workings of recombination
For many years, scientists lacked the experimental tools to see how this complex process works. But Hunter has helped complete this picture.
He developed methods to visualize the process of homologous recombination and decipher the precise choreography of molecular events that break DNA and then splice it back together to ensure that each sperm or egg cell gets a full set of chromosomes. He has also shown how some aspects of this process are disrupted by chemicals such as the widely used herbicide atrazine, which may intensify the effects of age-related fertility decline. As women age, their egg cells become more prone to chromosomal errors, increasing the risk of congenital disorders such as Down syndrome, in which a child is born with an extra chromosome 21.
It was for this body of work that Hunter was elected to the Royal Society.
The newly elected members’ “achievements represent the very best of scientific endeavor, from basic discovery to research with real-world impacts across health, technology and policy,” said Sir Adrian Smith, the President of the Royal Society. Founded in London in 1660, it is the world’s oldest continuously operating scientific academy, with Isaac Newton, Albert Einstein and Stephen Hawking among its former members.
Hunter finds all of this profoundly humbling. “To have the top scientists in the world saying that I can join this club — it’s really a great honor,” he said.
Hunter’s research is funded by the National Institutes of Health and the Howard Hughes Medical Institute. His work has also received funding from the American Cancer Society, the Concern Foundation for Cancer Research, and the Damon Runyon Cancer Foundation.
Hunter’s work on homologous recombination uses advanced scientific facilities at the University’s Proteomics Core Facility, MCB Light Microscopy Imaging Facility, Genome Center, Mouse Biology Program, and the Comprehensive Cancer Center.
Media Resources
- Hunter Lab
- Royal Society Announcement
- Oocyte Development and Quality in Young and Old Mice following Exposure to Atrazine (Environmental Health Perspectives 2022)
- PCNA activates the MutLγ endonuclease to promote meiotic crossing over (Nature 2020)
- A SUMO-ubiquitin relay recruits proteasomes to chromosome axes to regulate meiotic recombination (Science 2017)
- Meiotic Recombination: The Essence of Heredity (Cold Spring Harbor Perspectives in Biology 2015)
- Antagonistic roles of ubiquitin ligase HEI10 and SUMO ligase RNF212 regulate meiotic recombination (Nature Genetics 2014)
- Double Holliday junctions are intermediates of DNA break repair (Nature 2010)
- The Single-End Invasion: An Asymmetric Intermediate at the Double-Strand Break to Double-Holliday Junction Transition of Meiotic Recombination (Cell 2001)
