
Researchers Identify Microbes That Help Plants Thwart Parasite
Plant biologists have identified bacteria that help sorghum plants resist infections and could be used as a soil “probiotic”
Sorghum, or broomcorn, is a staple crop in sub-Saharan Africa, but approximately 20% of annual yields are lost due to infections with witchweed (Striga hermonthica), a parasitic plant that steals nutrients and water by latching onto the plant’s roots.
In a new study in Cell Reports published on March 26, University of California, Davis researchers show that soil microbes can suppress witchweed infections by inducing changes in sorghum root structure that make it more resistant to infection. The researchers identified specific strains of bacteria that trigger these resistance traits and could be applied as a soil “probiotic” to improve sorghum yields in future.
“These microbes have great promise as soil additives that can help farmers grow sorghum successfully in sub-Saharan Africa,” said Siobhan Brady, a professor in the Department of Plant Biology and Genome Center, and a senior author on the paper.

A witchy weed
Witchweed is a major issue for smallholder farmers in sub-Saharan Africa. The parasitic plants produce thousands of tiny seeds that can remain dormant in the soil for up to 20 years, making them extremely difficult to eradicate. Current control methods—which include applying chemical agents, crop rotation and breeding resistant sorghum—have only achieved partial control, and many are inaccessible to the farmers who need them most.
In the new study, postdoctoral scholar Dorota Kawa worked alongside Brady and collaborators in the Netherlands and Ethiopia to show that soil microbes can mitigate witchweed infections in sorghum.
“This is the first example showing how microbes can induce changes in host root cells that are associated with the suppression of witchweed,” said Brady.
Hijacking a plant signal
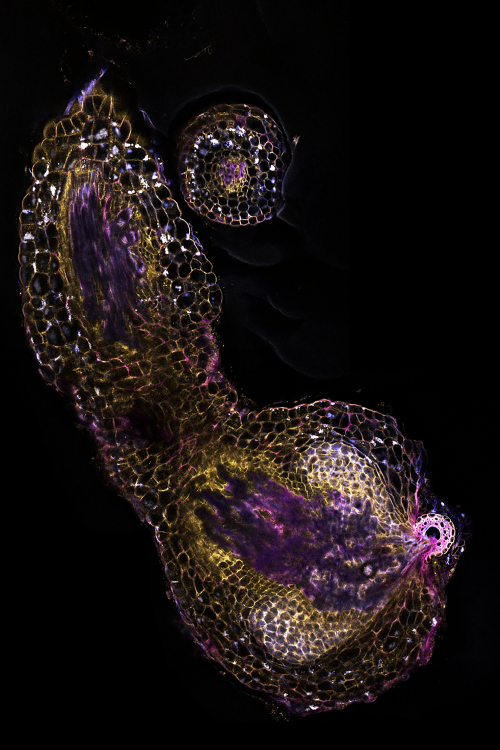
When sorghum plants find themselves in low-phosphate soil, they release chemicals from their roots to attract fungi to join them to help acquire phosphate. Unfortunately for sorghum, witchweed has evolved to respond to this same signal.
“This parasitic plant has hijacked the signaling so that its seeds germinate when they perceive that same signal from the root,” said Brady.
After germinating, witchweed responds to additional chemical cues from sorghum that trigger the parasite to grow appendages called “haustoria” that enable it to latch on and penetrate the sorghum roots.
“Once it has made this connection with the sorghum vasculature, it's like a superhighway of nutrients to the parasitic plant,” said Brady.
Brady and Kawa wanted to know whether soil microbes could interrupt this hijacking. Previous studies have shown that a species of soil fungus, Fusarium, suppresses witchweed germination, and thus infection of sorghum, but little is known about whether soil bacteria or fungi impact witchweed infections by changing the sorghum root.
As a first test, the researchers compared the susceptibility of sorghum seedlings that had been sprouted in “natural” soil to seedlings grown in sterilized, bacterially-depleted soil. When the researchers examined the sorghum plants several weeks later, they found that plants grown in natural soil had fewer witchweed hangers-on than those grown in sterilized soil, suggesting that bacteria play an important role in the plants’ ability to resist infection.
Microbial mechanisms
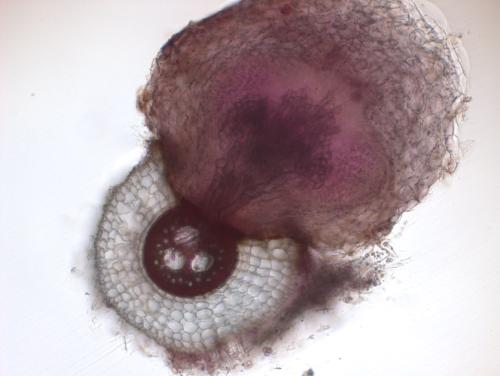
Next, the team wanted to investigate the mechanisms behind this resistance. Using a combination of genetics, microscopy and in vitro experiments, they showed that microbes degrade the chemical cues that help witchweed attach to its host and also alter the sorghum’s root anatomy to make it harder for witchweed to latch on. They observed that when sorghum plants are grown in natural, microbe-laden soil, the bacteria induce genes that result in a thicker layer of suberin, a waxy substance that may act as a barrier to witchweed, and more air-filled gaps or “aerenchymas” that may also impede witchweed’s attachment to sorghum.
Using genetic sequencing, Brady and her collaborators identified over 100 bacteria taxa that were associated with witchweed resistance. When they tested the functions of eight of these bacterial strains in vitro, they identified a strain of Pseudomonas bacteria that degrades chemical cues in the soil and a strain of Arthrobacter that increased root suberization in sorghum.
“It's exciting that we were able to identify individual microbes because typically you have a whole suite of microbes within the soil and it’s possible that they're acting together,” said Brady.

Another tool in the toolkit
“The ultimate goal is to identify microbial solutions that farmers can treat the soil or seeds with to help prevent Striga infection,” Brady said. “The intention is that this should be part of an integrated package of solutions to farmers—another tool in the toolkit.”
Now, the researchers are searching for microbes responsible for conferring the other resistance traits, such as aerenchyma formation. They’re also characterizing soil microbes from other regions, beginning with Ethiopia, and investigating whether these same microbes can confer witchweed resistance to other crop species that are also impacted by the parasite.
“We need to make sure that we're using microbes that come from the country in which those microbes will be applied so that we maintain biodiversity,” said Brady. “We also want to prioritize microbes that are able to work well in other crop species, like pearl millet and rice.”
Kawa is now an assistant professor at Utrecht University.
Additional authors on the study are: Tamera Taylor, Hannah Vahldick, Zayan Musa, Alexander Chen, and Jiregna Daksa, UC Davis; Desalegn Etalo, Utrecht University; Benjamin Thiombiano, Aimee Walmsley, Harro Bouwmeester, and Mario Schilder, Swammerdam Institute for Life; Mahdere Shimels, Dominika Rybka, Marcio Leite, Francisco Dini-Andreote, Eiko Kuramae, and Jos Raaijmakers, Netherlands Institute of Ecology; Alexander Bucksch, University of Georgia; and Taye Tessema, Ethiopian Institute of Agricultural Research.
The work was supported by the Bill and Melinda Gates Foundation, the Howard Hughes Medical Institute, the Advanced Research Projects Agency–Energy, and the National Science Foundation.
Media Resources
- The soil microbiome modulates the sorghum root metabolome and cellular traits with a concomitant reduction of Striga infection: Cell Reports
- Liana Wait is a freelance science writer based in Philadelphia. She has a Ph.D. in ecology and evolutionary biology and specializes in writing about the life sciences.
