Worms Reveal Just How Cramped Cells Really Are
UC Davis Research Shows Cytoplasm is less like a Pool than a Crowded Concert Venue
In a new study published in Science Advances on September 10, a team of UC Davis researchers tracked the movement of fluorescent particles inside the cells of microscopic worms, providing unprecedented insights into cellular crowding in a multicellular animal. They found that the cytoplasm inside the worms was significantly more crowded and compartmentalized than in single-celled yeast or mammalian tissue culture cells, which are more commonly used to gauge internal cellular dynamics.
This difference highlights the importance of studying cellular processes in living animals rather than cell culture, says first author Xiangyi Ding, a Ph.D. candidate in the Integrative Genetics and Genomics graduate group.
“This changes everything. Crowding in a cell affects any process that depends on molecule movement and interaction, including drug delivery, disease progression, and how cells respond to stress.”

Worm cells paint a different picture
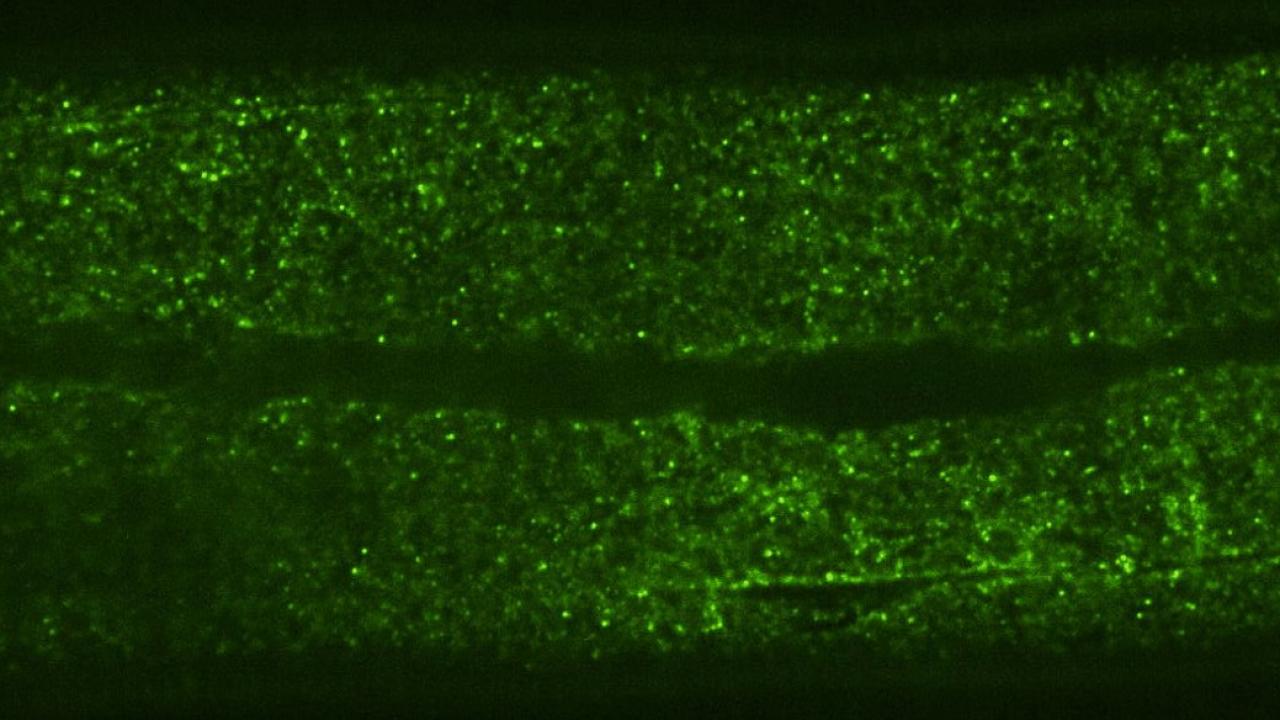
To investigate how particles move inside multicellular organisms, the team, co-led by Daniel Starr, a professor of molecular and cellular biology, and G.W. Gant Luxton, an associate adjunct professor of molecular and cellular biology, used protein structures called Genetically Encoded Multimeric Nanoparticles (GEMs). GEMs are based on naturally occurring proteins from archaea that self-assemble into particles that are around 40 nm in diameter, about the size of a ribosome. The GEMs have been altered so that their surfaces are covered in fluorescent tags, which allows their movements to be tracked under the microscope at a rate of up to 50 frames per second.
The researchers inserted the DNA instructions for GEMs into the genome of Caenorhabditis elegans, a microscopic nematode with transparent skin whose internal structures can be easily imaged. The resulting worms developed and behaved normally, but their intestinal and skin cells produced thousands of fluorescently tagged GEMs.
Using time-lapse microscopy videos, the researchers found that, on average, the GEMs moved around 50 times more slowly in C. elegans cells than in cultured mammalian or single yeast cells. They also observed that most GEMs were not only crowded, but restricted to certain areas, suggesting that something was compartmentalizing the cells.
“When we first noticed that the worm cells were very constrained, we thought it was a mistake, because this is completely different from what is seen in yeast or mammalian tissue culture cells, which are not constrained,” said Ding.
What controls cellular compartmentalization?
Next, the team wanted to understand how the worm cells maintained this more orderly environment. They started by investigating the role of a large protein called ANC-1 that helps maintain cellular architecture by acting as a scaffold. When they disrupted ANC-1 production, the worms’ cells were just as crowded as before, but the GEMs were no longer constrained to certain parts of the cytoplasm.
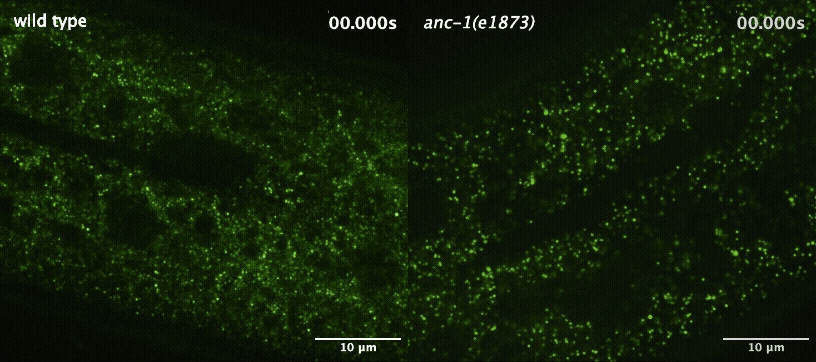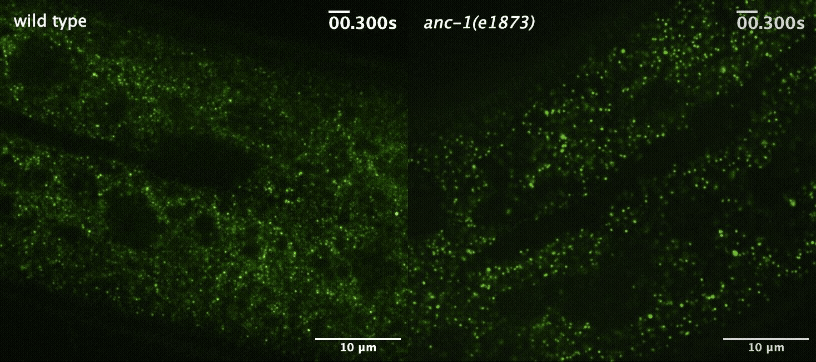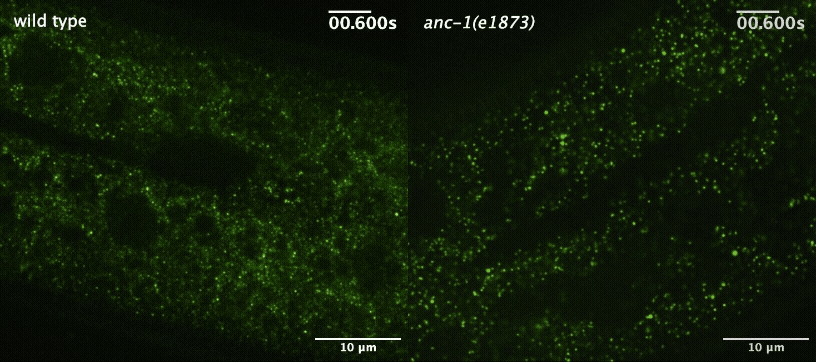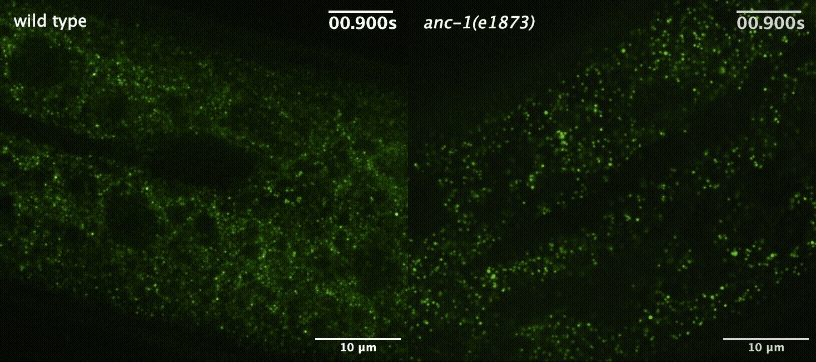
Cytoplasmic crowding, on the other hand, was controlled by the concentration of ribosomes — the same protein-producing structures that control crowding in yeast and tissue-cultured mammalian cells. And when the team disrupted the production of both ANC-1 and ribosomes, the GEMs’ movement became much faster and less restricted.
“This shows that cells use two complementary systems to control particle mobility,” said Luxton. “The ribosomes are acting like packing peanuts in a box, and the boxes themselves may be the ANC-1 protein complexes. We already knew that ribosomes were acting like packing peanuts, but until now, we didn't understand how these two pathways worked together.”
The next frontier: studying biophysics in living animals
Getting GEMs to work in the worms was a challenge that involved years of work. “It ended up being more difficult than even we imagined,” said Starr. “However, this study highlights the importance of studying cells in living organisms rather than cell culture, because the physical environment of a tissue-cultured cell is so different from anything in an actual organism.”
Now that they’ve developed a system for using GEMs in multicellular animals, the team is excited to investigate other cell types within the worms, such as neurons, to understand how the cytoplasm changes during aging and neurodegeneration. They also plan to use GEMs to investigate particle movement in more complex organisms, starting with zebrafish.
“This is the next frontier,” said Luxton. “We're trying to figure out how the physical properties of cells contribute to health and disease, and the only way we can do this is by looking at tissues in living organisms the intersection of developmental genetics and cellular biophysics.”

Additional authors on the study are: Hongyan Hao, Daniel Elnatan, Patrick Neo Alinaya, Shilpi Kalra, Abby Kaur, and Sweta Kumari; UC Davis; and Liam Holt, NYU Grossman School of Medicine.
The work was supported by the National Institutes of Health and an Allen Distinguished Investigator Award from the Paul G. Allen Frontiers Group of the Paul G. Allen Family Foundation. This research utilized the MCB Light Microscopy Imaging Facility.
Media Resources
- Giant KASH proteins and ribosomes establish distinct cytoplasmic biophysical properties in vivo
- Liana Wait is a freelance science writer based in Philadelphia. She has a Ph.D. in ecology and evolutionary biology and specializes in writing about the life sciences.
