Wily Parasite Kills Human Cells and Wears Their Remains as Disguise
The single-celled parasite Entamoeba histolytica infects 50 million people each year, killing nearly 70,000. Usually, this wily, shape-shifting amoeba causes nothing worse than diarrhea. But sometimes it triggers severe, even fatal disease by chewing ulcers in the colon, liquefying parts of the liver and invading the brain and lungs.
“It can kill anything you throw at it, any kind of human cell,” said Katherine Ralston, an associate professor in the Department of Microbiology and Molecular Genetics. E. histolytica can even evade the immune system – and it can kill the white blood cells that are supposed to fight it.
Scientists have struggled to understand how it does this. But in a new paper published in the May issue of Trends in Parasitology, Ralston and her lab lay out a plan for finally tackling it, using genetic tools to tease apart the function of its proteins and genes.
“All parasites are understudied, but E. histolytica is especially enigmatic,” said Ralston. Building the tools for studying it has taken years. But along the way she has uncovered surprises that may set the stage for improved treatments.
Finding the murder weapon of a microscopic killer
E. histolytica enters the colon after a person ingests contaminated food or water, generally in developing countries with poor water sanitation. In the United States, it is most commonly seen in people who recently took trips overseas, or who immigrated from other countries.
Its species name, histolytica, means “tissue-dissolving” — because it creates festering pockets of liquefied tissue, called abscesses, in the organs it infects. As it rampages through a person’s organs, it doesn’t neatly eat the cells that it kills; instead, it leaves the wounded cells to spill out their contents while it hurries on to kill other cells.
Ralston started studying this fearsome little beast in 2011, during a postdoctoral fellowship at the University of Virginia. Back then, people believed that it killed cells by injecting them with a poison. But as she watched it through a microscope, she saw something very different.
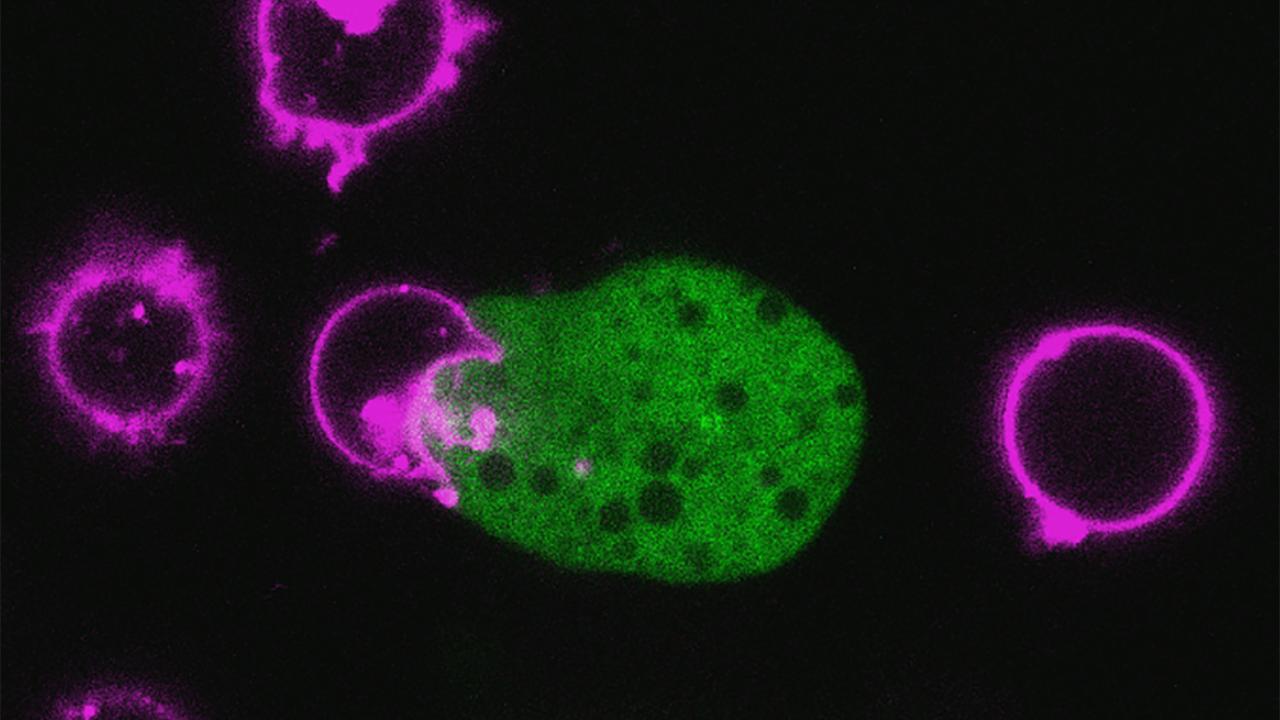
E. histolytica was actually taking bites out of human cells. Peering through the microscope, “You could see little parts of the human cell being broken off,” she said. Those ingested cell fragments, shining fluorescent green under her microscope, accumulated inside the amoeba.
Her report that the parasite kills cells through this process, called “trogocytosis,” was published in the journal Nature in 2014. “This was important,” she said. “To devise new therapies or vaccines, you really need to know how E. histolytica damages tissue.”
Ralston discovered, in 2022, that after the amoeba ingests parts of human cells, it becomes resistant to a major component of the human immune system — a class of molecules called “complement proteins” that finds and kills invading cells.
In a new paper, posted to bioRxiv in October 2024, Ralston and graduate students Maura Ruyechan and Wesley Huang found that the amoeba gains this resistance by ingesting proteins from the outer membranes of human cells and placing them on its own outer surface. Two of these human proteins, called CD46 and CD55, prevent complement proteins from latching onto the amoeba’s surface.
In essence, the amoebae are killing human cells and then donning their protein uniforms as a disguise, allowing them to evade the human immune system.
Building tools for scientific discovery
With other pathogens, like HIV and salmonella, scientists made rapid progress by identifying many genes, then running high-throughput experiments to knock those genes out individually to find ones that are crucial for causing disease. But that has been difficult with E. histolytica.
Its genome, sequenced in 2005, is five times larger than that of salmonella and 2,500 times larger than that of HIV. Analyzing it required years of advances in bioinformatics. But a 2013 study finally showed something promising: E. histolytica uses a cellular process called “RNA inhibition” (RNAi) as a volume knob, to control the expression of its genes.
“We thought we could turn this into a tool for understanding its genome,” Ralston said. In 2021 she, Ruyechan, Huang and six UC Davis colleagues published a paper demonstrating one such tool that they developed. Their “RNAi library” allows them to inhibit the expression, individually, of each one of the parasite’s 8,734 known genes.
In their newest paper, published as the cover story this month in Trends in Parasitology, Ralston, Huang and Ruyechan present a plan for using this RNAi system to quickly identify genes that are required for the amoeba to do crucial things, like biting human cells or stealing their proteins.
They advocate for combining this with the gene-editing tool CRISPR. Using this approach, they could label proteins with fluorescent markers, to watch them interact under a microscope; or delete small parts of genes and proteins, to find the specific parts that are crucial and could be targeted with drugs.
“We now see a light at the end of the tunnel, and we think this could be achievable,” said Huang, who is advocating for the research community to develop CRISPR for use in the amoeba.
From basic research to medical breakthroughs
The story of E. histolytica illustrates the value of basic research — and how it contributes to medical breakthroughs many years down the road.
It took years for researchers to learn how to grow this parasite in the lab and years more for Ralston to discover how it bites human cells and coopts their proteins to escape the immune system. Even after its genome was sequenced, Ralston and other scientists needed time to develop experimental tools for picking it apart. This lays the foundation for identifying genes or proteins that can be targeted with vaccines or new drugs.
“Science is a process of building,” Ralston said. “You have to build one tool upon another, until you’re finally ready to discover new treatments.”
Ralston’s work has been funded by the National Institutes of Health and received support from the American Society for Microbiology, Pew Charitable Trusts, the Hellman Foundation, and the Hartwell Foundation. This research has utilized several UC Davis research core facilities, including the Light Microscopy Imaging Facility, the Bioinformatics Core Facility, and the Center for Molecular and Genomic Imaging.
Additional authors of the 2021 paper presenting the RNAi library include: Akhila Bettadapur, Rene Suleiman, Hannah Miller and Tammie Tam (UC Davis College of Biological Sciences); Samuel Hunter and Matthew Settles (UC Davis Genome Center); and Charles Barbieri (SeqMatic LLC, Fremont, CA).

Media Resources
- Douglas Fox is a freelance science writer based in the Bay Area.
- Work with me here: variations in genome content and emerging genetic tools in Entamoeba histolytica (Trends in Parasitology, May 2025)
- Ralston Laboratory
- Katherine Ralston, Microbiology and Molecular Genetics, ksralston@ucdavis.edu
- Andy Fell, News and Media Relations, 530-304-8888, ahfell@ucdavis.edu
