Defects in DNA Packaging May Drive Age-Related Decline in Fertility
Prestigious new GCRLE grant will fund research into the epigenetic origins of reproductive aging
Yasuhisa Munakata, a postdoctoral fellow in the College of Biological Sciences, has received a grant to study how egg cells in the ovary change over time. “Our goal is to understand female reproductive aging, and why fertility rapidly declines starting in the mid-30s,” says Satoshi Namekawa, a professor of Microbiology and Molecular Genetics, in whose lab Munakata works.
The grant from the Global Consortium for Reproductive Longevity and Equity (GCRLE) was announced in June. It will provide $200,000 in funding, spread over two years. With it, Munakata will examine a little-known aspect of reproductive biology: the way an egg cell stops growing and enters a holding pattern, right around the time a female is born. At this point, the cell’s DNA is packaged with a matrix of specialized proteins – leaving it primed for the crucial moment, decades later, when the egg is fertilized and begins growing into an embryo.
“We don’t know how these cells are maintained for decades,” says Namekawa. “We want to know the secret of this mechanism.” He and Munakata believe that gradual changes in the way the DNA is stored could lead to a loss of fertility as women age. If so, their research could pave the way for improved treatment and diagnosis of fertility problems.
Keeping DNA untangled and organized
By the time a human female is born, her developing ovaries already contain all of the egg cells, or “oocytes,” that she will ovulate over the course of her reproductive life. Each one contains a mass of thread-like DNA — akin to 400 miles of yarn. To keep it from devolving into a tangle, the DNA is wrapped around a series of protein spools, called nucleosomes.
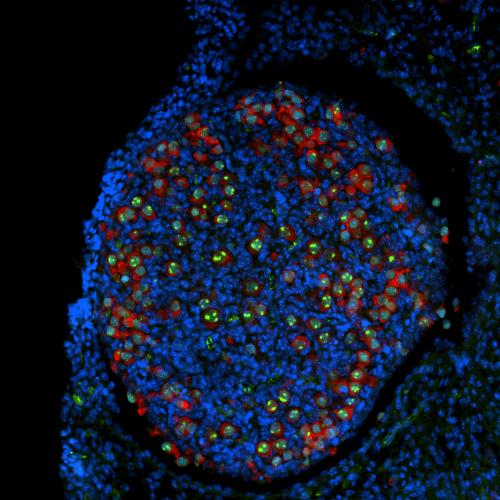
The DNA in a human oocyte encompasses some 15 million nucleosomes — wrapping twice around each one — forming an organized structure called chromatin.
“The organization of chromatin influences which genes can be expressed,” says Namekawa. That’s because the specific locations of the spools, and the spacing between them, determines which stretches of DNA can be accessed by molecules called transcription factors, which latch onto genes and turn them on. Large protein machines, called chromatin remodelers, can shift the positions of spools up and down the DNA thread, thereby facilitating the expression — or silencing — of thousands of genes.
For an oocyte to be ready for fertilization and growth into an embryo, the right set of genes needs to be turned on. Namekawa suspected that this pre-programming of the oocyte genome happens in several stages — the first one starting around the time a female is born. He believed that chromatin remodeling proteins did much of this work, by shifting spools around.
In 2022, they discovered that a set of proteins called Polycomb repressive complex 1 (PRC1) helps silence hundreds of genes around the time of birth by modifying the structure of spools. This allows the oocytes to pause their growth and enter a holding pattern, which lasts into the mouse’s adulthood. In mice lacking PRC1, the oocytes didn’t pause their growth; instead, they withered and died within a month, leaving the mice permanently infertile.
Genomic choreography
Using the GCRLE grant, Munakata and Namekawa will look more closely at how oocytes are genomically primed and stored for later use. They will investigate the role of a chromatin remodeler in oocyte survival and fertility. This protein may “establish and maintain the chromatin state,” says Namekawa — keeping the spools in place and the oocytes genetically primed as the mouse matures.
Munakata and Namekawa will compare mice with normal and mutated versions of the chromatin remodeler. As the mice grow and mature, they will observe whether the presence or absence of this protein triggers differences in the arrangement of DNA spools in the animals’ oocytes. And they will monitor whether this, in turn, alters the pattern of gene expression over time.
Namekawa believes that in human oocytes, changes in these patterns could disrupt the delicate choreography of genes being turned on and off at the proper times — leaving them unprepared for fertilization or growth into healthy embryos. Changes in chromatin structure as an oocyte ages might also impact the way that chromosomes segregate during cell division. This could help explain why older women are more likely to bear children with chromosomal disorders like Down Syndrome, in which an extra copy of chromosome 21 finds its way into an oocyte — and later, an embryo.
“If we know how this chromatin state is established, and how it relates to oocyte quality, it could eventually lead to therapies for some types of infertility,” says Namekawa.
This work has been funded by grants from the National Institutes of Health, in addition to the new grant from the Global Consortium for Reproductive Longevity and Equity.
Media Resources
- Douglas Fox is a freelance science writer based in the Bay Area.
- PRC1-mediated epigenetic programming is required to generate the ovarian reserve (Nature Communications 2022)
- Namekawa Lab
- Global Consortium for Reproductive Longevity and Equality, 2023 Grantees
