
Understanding Neutrophil Decision-Making: How Immune Cells Prioritize Competing Signals
By switching off lower-priority receptors, the cells concentrate on higher-priority threats like infection
Neutrophils, the primary foot soldiers of the immune system, swarm to sites of infection and inflammation by following breadcrumb pathways made up of signaling molecules. But the human body is a complex place, and neutrophils are often simultaneously bombarded with multiple signals, some of which are more important than others. For example, signals of infection or tissue damage require more urgent attention than signals produced by other immune cells.
So how do neutrophils prioritize where to swarm when faced with competing calls to action? Studies have shown that neutrophils are capable of identifying and prioritizing higher-priority signals, but the mechanism behind how they make these choices was unclear. Understanding this process could have future applications in mitigating excess inflammation, which is a hallmark of many diseases, including COVID-19.
A new study from researchers in College of Biological Sciences shows that one way that neutrophils achieve prioritization is by switching off responses to lower-priority signals while sustaining responses to high-priority signals. This allows them to appropriately focus their responses when multiple threats are present. The study was published Oct. 3 in the journal Science Signaling.

“This prioritization allows neutrophils to zero-in on the right target,” said Sean Collins, an associate professor in the Department of Microbiology and Molecular Genetics and senior author on the paper. “This mechanism creates a way for high-priority signals to overpower lower-priority signals while still allowing those signals to work if they're the only signal present,” said Collins.”
The study was launched by one of Collins’s first graduate students, Briana Rocha-Gregg, after a conversation at the annual retreat for the NIH-funded Training Program in Molecular and Cellular Biology. Stefan Lundgren, also a Ph.D. student, joined the team later and led the completion of the project.
Uncovering how neutrophils prioritize competing signals
The team used live cell imaging and fluorescent markers to compare how cells responded to a high-priority signal versus a lower-priority signal. Compared to previous studies that were only able to measure cellular responses every few minutes, this method allowed the researchers to monitor the cells’ activity on a second-by-second basis - as well as to measure the responses of individual cells.
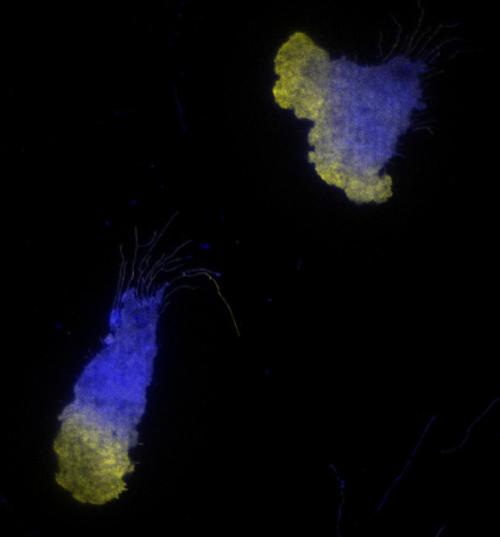
They found that though both low- and high-priority signals elicited responses of similar magnitude in the short term, responses to low-priority signals were much more transient. Neutrophils remained activated for more than two minutes when stimulated with a high-priority signal, but for only 20-40 seconds when stimulated with a low-priority signal.
“At short time scales, the strength of the response is the same for both types of signals, so it's not that one is bigger than the other,” Collins said. “The difference is really in the duration: the response to the lower-priority signal shuts off very quickly.”
Switching off the signal
By genetically manipulating the receptor that detects low-priority signals, the researchers were able to pinpoint part of the mechanism behind its rapid switching off. They identified two sites on the low-priority receptor that are altered via phosphorylation soon after the receptor is activated, which weakens the receptor’s ability to interact with other molecules and effectively switches the signal off.
“There are clearly differences between the receptors that recognize these different signals,” said Collins. “We found one of them, but we think there are more, and we would really like to make a more complete map of what differentiates receptors for low- and high-priority signals.”
Linking molecular mechanisms to cell swarming behavior
Next, the researchers tested whether these molecular-scale changes impacted how neutrophils behave when faced with competing signals.
“The first part of the study allowed us to see how a cell responds when it sees one signal versus the other, and how the responses are different at the molecular level,” said Collins. “The second part was putting it together at the level of cell behavior and asking if those molecular differences are really linked to the swarming behavior that we're interested in.”
They found that the differences did indeed translate to altered cell behavior. When given the choice, neutrophils prioritized migration towards the high-priority signal. However, when the cells were mutated so that they were unable to “switch off” the low-priority signaling, they migrated in a more random manner and failed to prioritize the high-priority signal.
Understanding the mechanisms behind neutrophil swarming could have future applications in mitigating certain diseases. “It is a really important process for protecting the body, but also one that frequently goes too far and causes problems,” Collins said. “One of the end goals would be to figure out new strategies for limiting inflammatory disease.”
Coauthors on the paper are Stefan Lundgren, Briana Rocha-Gregg, Emel Akdoǧan, Maya N. Mysore, and Samantha Hayes of the Department of Microbiology and Molecular Genetics.
The work was supported in part by grants from the National Institutes of Health, the Sidney Kimmel Foundation, the Floyd and Mary Schwall Fellowship, the Floyd and Mary Schwall Dissertation Year Fellowship, and the Lurie Graduate Student Awards.
Media Resources
- Liana Wait is a freelance science writer based in Philadelphia. She has a Ph.D. in ecology and evolutionary biology and specializes in writing about the life sciences.
- Signaling dynamics distinguish high- and low-priority neutrophil chemoattractant receptors (Science Signaling)
